BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
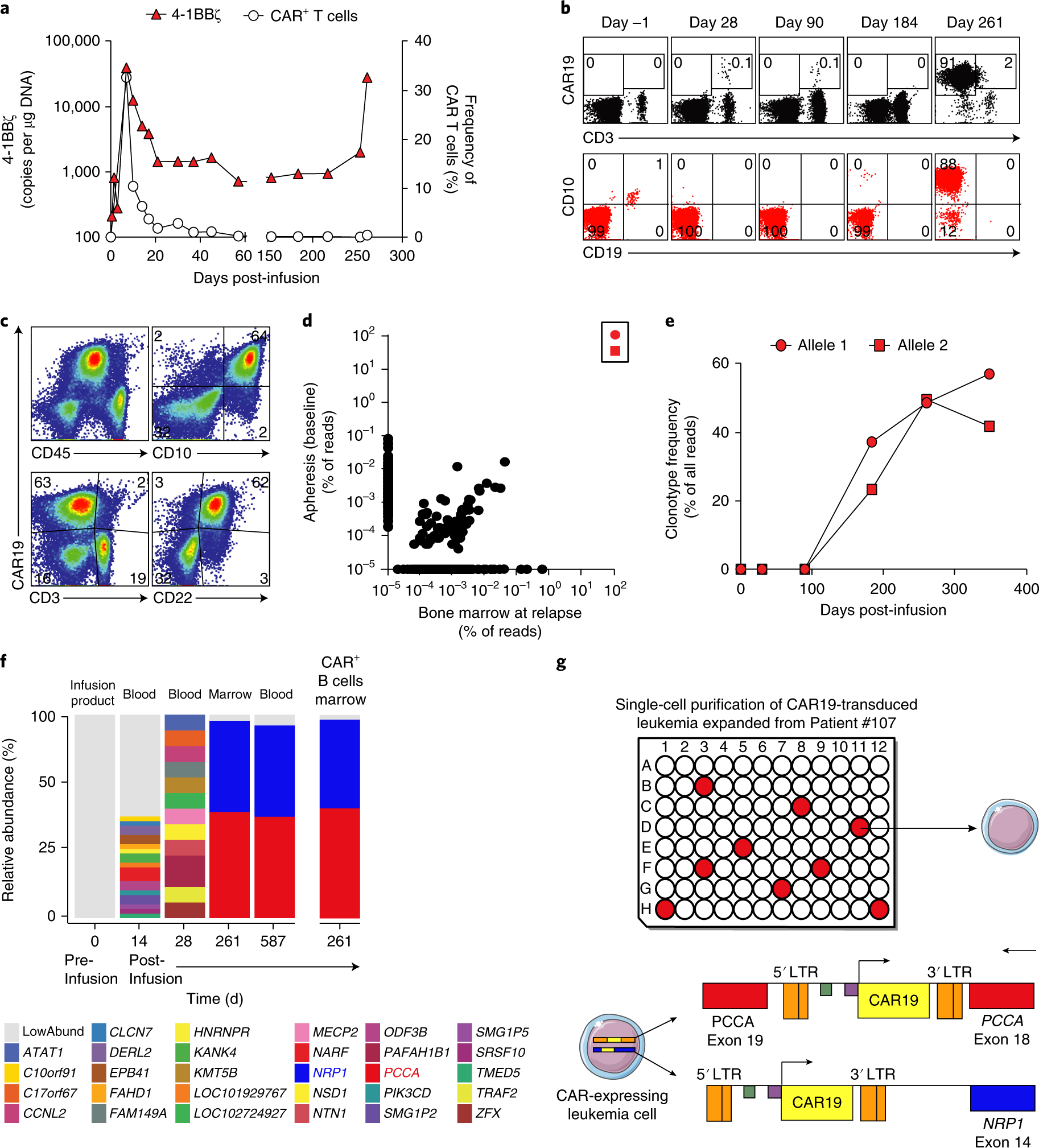
Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell | Nature Medicine

A New Approach to Treat Childhood Leukemia: Novartis' CAR-T Therapy - Frazer A. Tessema, Jonathan J. Darrow, 2017
A New Approach to Treat Childhood Leukemia: Novartis' CAR-T Therapy | Journal of Law, Medicine & Ethics | Cambridge Core

Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials: Molecular Therapy - Methods & Clinical Development

The long road to the first FDA-approved gene therapy: chimeric antigen receptor T cells targeting CD19 - Cytotherapy
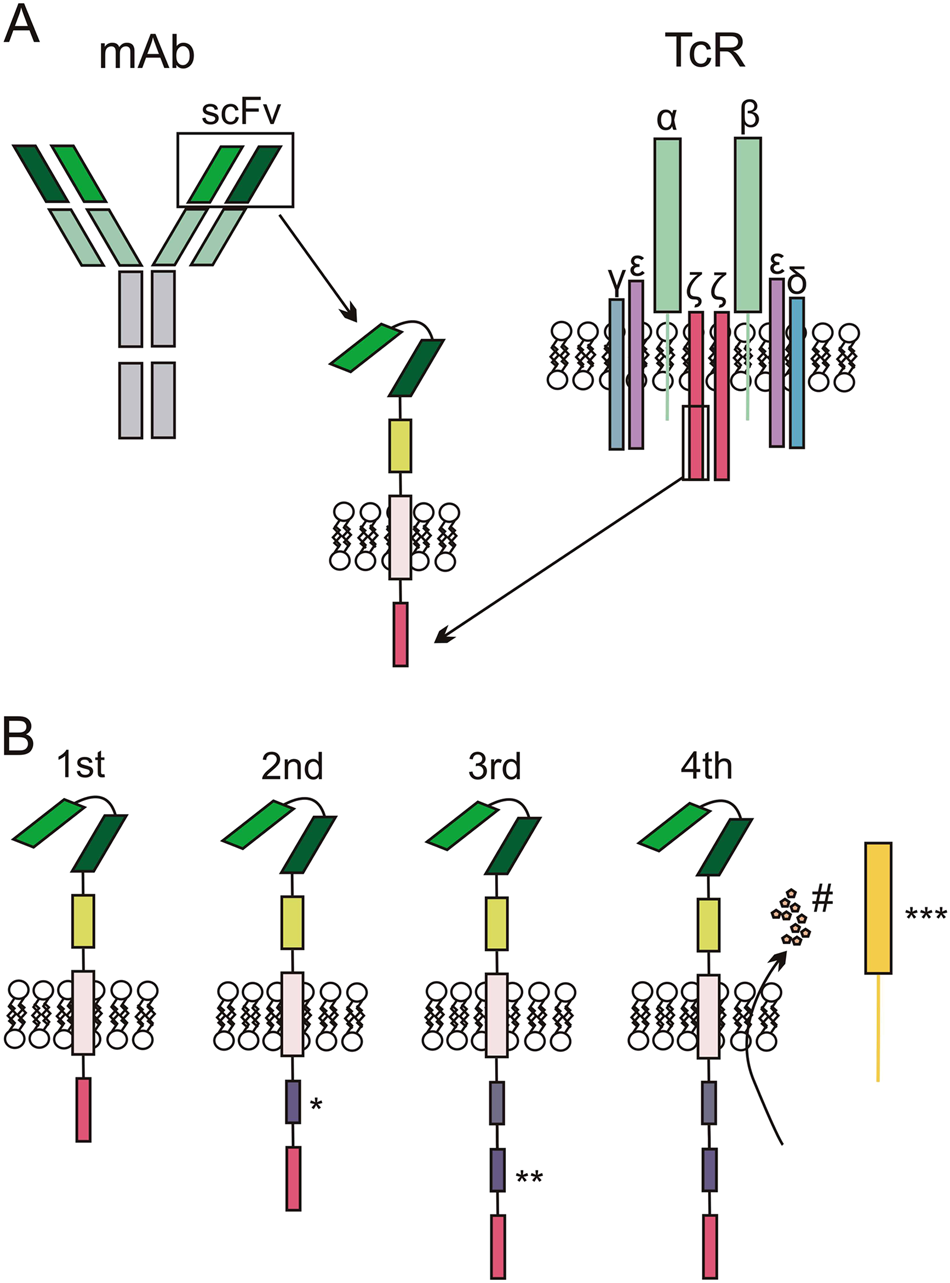
The biological basis and clinical symptoms of CAR-T therapy-associated toxicites | Cell Death & Disease
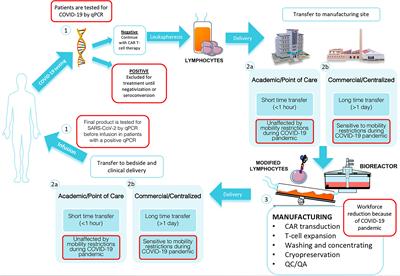
Frontiers | Manufacturing and Management of CAR T-Cell Therapy in “COVID-19's Time”: Central Versus Point of Care Proposals
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
ONCOLOGIC DRUGS ADVISORY COMMITTEE BRIEFING DOCUMENT Tisagenlecleucel (CTL019) for the TREATMENT OF PEDIATRIC AND YOUNG ADULT PA
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar

Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials: Molecular Therapy - Methods & Clinical Development

Can CAR-T and gene therapy cures really sustain biopharma? Not for all, analyst says | Fierce Pharma

Characteristics of anti-CD19 CAR T cell infusion products associated with efficacy and toxicity in patients with large B cell lymphomas | Nature Medicine